Saccharin

Saccharin is a high intensity, artificial sweetener that has been used for over one hundred years as a sugar substitute. Saccharin tastes over 500 times sweeter than sugar which means that it can be used in small amounts to reduce sugar consumption.
Saccharin has no calories and a Glycemic Index (GI) of zero. Saccharin is not absorbed or broken down by the body and has no effect on blood sugar levels. It is therefore considered as an important sugar substitute to help combat diabetes and obesity. Saccharin is also heat stable. Under conditions of increasing heat, saccharin remains stable at temperatures up to at least 250°C. Therefore, saccharin is commonly used in candies, cookies, some formulations of soft drinks as well as in mouth washes, toothpastes and as part of the tablet coating in medicines. We also produce the saccharin that is used to make table top sweeteners.
| Product | Structural Formula | Downloads | Use |
|---|---|---|---|
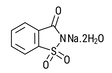
| Sodium Saccharin 15% moisture CAS # 6155-57-3 CAS # 82385-42-0 CAS # 128-44-9 |
 |
Specification Sheet |
|
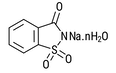
| Sodium Saccharin 6% moisture CAS # 6155-57-3 CAS # 82385-42-0 CAS # 128-44-9 |
 |
Specification Sheet | |
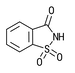
| Insoluble Saccharin CAS # 81-07-2 |
 |
Specification Sheet |
Calorie Control Council : https://caloriecontrol.org/saccharin/
Saccharin : https://saccharin.org